Density Worksheet
1.
A student has a measuring cylinder and a small, irregularly shaped piece of metal. The piece of metal can easily fit into the measuring cylinder.
Describe how the student can use the measuring cylinder and some water to find the volume of the metal.
..................................................................................................................................................
..................................................................................................................................................
[Total: 4]
2.
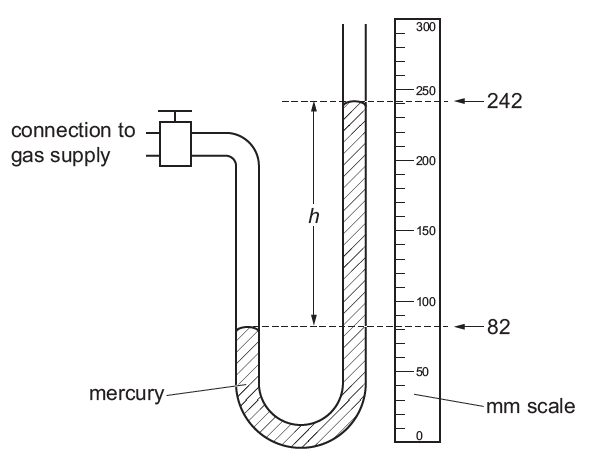
A device for measuring gas pressure is connected to a gas supply as shown in the diagram.
Suggest why this measuring device uses mercury rather than coloured water.
..................................................................................................................................................
[Total: 1]
3.
The volume of a block is 0.0089 m3. The mass of the block is 70 kg.
Calculate the density of the block.
density = .............................................. kg/m3
[Total: 3]
4.
A bowl contains 500 cm3 of water at a temperature of 5.0 °C. The bowl of water is placed in a freezer for several hours. When the bowl is removed from the freezer, it contains ice at a temperature of –18.0 °C. The density of water is 1000 kg/m3.
(a) Calculate the mass of water in the bowl when it is placed in the freezer.
mass = ..............................................
(b) The specific heat capacity of water is 4200 J/(kg °C). The specific heat capacity of ice is 2100 J/(kg °C). The specific latent heat of fusion of water is $3.3 \times 10^5$ J/kg.
Calculate the energy given out as the water cools from 5.0 °C to ice at –18.0 °C.
energy = ..............................................
[Total: 7]
5.
A student has a measuring cylinder, a beaker of liquid and a balance.
Describe how the student can use this equipment to determine the density of the liquid.
..................................................................................................................................................
..................................................................................................................................................
..................................................................................................................................................
..................................................................................................................................................
..................................................................................................................................................
[Total: 3]
6.
A metal block has a mass of 86 g and a volume of 8.0 cm3.
(a) Calculate the density (\ \rho \) of the metal using the equation
density of metal = .............................................. g/cm3
(b) The metal block is placed in some liquid. The metal block floats on the liquid.
Suggest a value for the density of the liquid.
............................................... g/cm3
[Total: 3]
7.
A sample of sand has a volume of 0.050 m3. The density of the sand is 1900 kg/m3. The specific heat capacity of the sand is 1500 J/(kg °C).
(a) Calculate the mass of the sample of sand.
mass = .....................................................
(b) Calculate the thermal capacity of the sample of sand.
thermal capacity = .....................................................
[Total: 4]
8.
A stone has a mass of 98.4 g. The volume of this stone is 41.0 cm3.
Calculate the density of the stone.
density = .............................................. g/cm3
[Total: 3]
9.
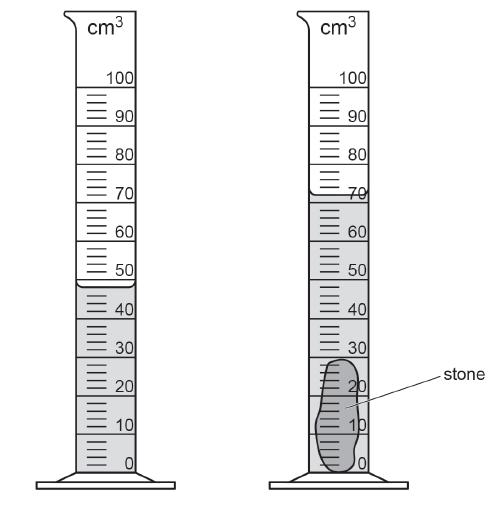
The diagram shows a measuring cylinder before and after having a stone lowered into it.
Calculate the volume of the stone.
volume = .......................................... cm3
[Total: 2]
10.
In an experiment, a metal block is heated and the temperature of the metal block increases by 100 °C.
State the effect, if any, of the temperature increase on:
- the volume of the metal block ..............................................................................................
- the mass of the metal block .................................................................................................
- the density of the metal block ..............................................................................................
[Total: 3]
11.
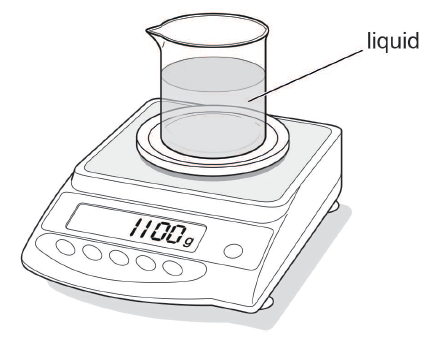
The diagram shows a beaker containing liquid on a top pan balance.
The mass of the empty beaker is 400 g.
(a) Using the information in the diagram, determine the mass of the liquid in the beaker.
mass = .............................................. g
(b) The beaker contains 750 cm3 of liquid.
Calculate the density of the liquid.
density = .............................................. g/cm3
[Total: 4]
12.
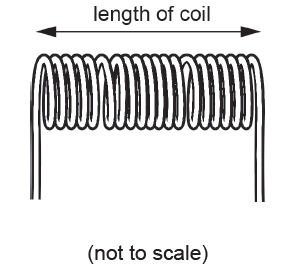
The diagram shows a coil of wire.
The volume of the wire in the coil is 16.6 cm3 and its mass is 148 g.
Calculate the density of the metal used for the wire in the coil.
density = .............................................. g/cm3
[Total: 3]